Medical Applications of NIR Spectroscopy

In recent years, near-infrared (NIR) spectroscopy has seen much progress in instrumentation and measurement techniques. It has been used for monitoring in many fields of analytical spectroscopy. Examples are the characterization of materials from processes of the chemical and pharmaceutical industry in addition to the broad field of food industrial and biotechnological applications. Another important field for NIR spectroscopy is found within the medical sciences with topics such as clinical chemistry, sensing and monitoring of changes of homeostasis of the body, with biofluids and tissues from many organs involved. Here, in vitro laboratory work and in vivo monitoring must be mentioned. Regarding instrumentation, laboratory analyzers are kept firmly in our view, but point-of-care (POC) applications need also to be taken into account. Sensing devices for non-invasive measurements on special parameters such as blood glucose and hemoglobin or information on the redox status of tissues is another broad area with oxygenation of hemoglobin and myoglobin as most important parameters. Absorption measurements are most often carried out with transmission and reflection techniques, but due to the synthesis of new marker substances, also fluorescence measurements in the NIR spectral range become more advanced, especially for imaging and immunoassay developments.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save
Springer+ Basic
€32.70 /Month
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Buy Now
Price includes VAT (France)
eBook EUR 71.68 Price includes VAT (France)
Softcover Book EUR 89.66 Price includes VAT (France)
Hardcover Book EUR 158.24 Price includes VAT (France)
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others

Clinical Application of NIRS
Chapter © 2013

Near-Infrared Spectroscopy for Noninvasive Measurement of Blood Glucose: Problems, Progress, Tasks
Article 12 May 2022
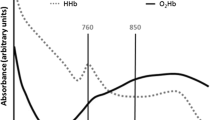
Review of early development of near-infrared spectroscopy and recent advancement of studies on muscle oxygenation and oxidative metabolism
Article 29 July 2019
References
- F.F. Jöbsis, Non-invasive infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 198, 1264–1267 (1977) ArticlePubMedGoogle Scholar
- D.T. Delpy, M. Cope, Quantification in tissue near-infrared spectroscopy. Philos. Trans. R. Soc. Lond. B. 352, 649–659 (1997). https://doi.org/10.1098/rstb.1997.0046ArticleCASGoogle Scholar
- B. Chance, E. Anday, S. Nioka, S. Zhou, L. Hong, K. Worden, C. Li, T. Murray, Y. Ovetsky, D. Pidikiti, R. Thomas, A novel method for fast imaging of brain function, non-invasively, with light. Opt. Express 2(10), 412–423 (1998) ArticleGoogle Scholar
- J.E. Bertie, Z. Lan, Infrared intensities of liquids XX: The intensity of the OH stretching band of liquid water revisited, and the best current values of the optical constants of H2O(l) at 25°C. Appl. Spectrosc. 50, 1047–1057 (1996) ArticleCASGoogle Scholar
- R.A. Shaw, H.H. Mantsch, Multianalyte serum assays from mid-IR spectra of dry films on glass slides. Appl. Spectrosc. 54(6), 885–889 (2000) ArticleCASGoogle Scholar
- A.K. Amerov, J. Chen, M.A. Arnold, Molar absorptivities of glucose and other biological molecules in aqueous solutions over the first overtone and combination regions of the near-infrared spectrum. Appl. Spectrosc. 58(10), 1195–1204 (2004) ArticleCASPubMedGoogle Scholar
- R. Marbach, T. Koschinsky, F.A. Gries, H.M. Heise, Noninvasive blood glucose assay by near-infrared diffuse reflectance spectroscopy of the human inner lip. Appl. Spectrosc. 47, 875–881 (1993) ArticleCASGoogle Scholar
- H.M. Heise, R. Marbach, A. Bittner, T. Koschinsky, Clinical chemistry and near-infrared spectroscopy: multicomponent assay for human plasma and its evaluation for the determination of blood substrates. J. Near. Infrared. Spectroscopy. 6, 361–374 (1998) ArticleCASGoogle Scholar
- Heise HM, Lampen P, Marbach R (2009) Near-infrared reflection spectroscopy for non-invasive monitoring of glucose – Established and novel strategies for multivariate calibration. In: Handbook of Optical Sensing of Glucose in Biological Fluids and Tissues, Tuchin VV (ed.), CRC Press, Chapter 5, 115–156 Google Scholar
- Perez-Guaita D, Garrigues S, de la Guardia M (2014) Infrared-based quantification of clinical parameters, Trends. Anal. Chem. 62 (2014) 93–105] Google Scholar
- K.Z. Liu, M. Shi, A. Man, T.C. Dembinski, R.A. Shaw, Quantitative determination of serum LDL cholesterol by near-infrared spectroscopy. Vib. Spectrosc. 38, 203–208 (2005) ArticleCASGoogle Scholar
- K.H. Hazen, M.A. Arnold, G.W. Small, Measurement of glucose and other analytes in undiluted human serum with near-infrared transmission spectroscopy. Anal. Chim. Acta 371, 255–267 (1998) ArticleCASGoogle Scholar
- M. Ren, M.A. Arnold, Comparison of multivariate calibration models for glucose, urea, and lactate from near-infrared and Raman spectra. Anal. Bioanal. Chem. 387, 879–888 (2007) ArticleCASPubMedGoogle Scholar
- L. Liu, M.A. Arnold, Selectivity for glucose, glucose-6-phosphate, and pyruvate in ternary mixtures from the multivariate analysis of near-infrared spectra. Anal. Bioanal. Chem. 393, 669–677 (2009) ArticleCASPubMedGoogle Scholar
- R.D. Whitehead Jr., Z. Mei, C. Mapango, M.E.D. Jefferds, Methods and analyzers for hemoglobin measurement in clinical laboratories and field settings. Ann. N. Y. Acad. Sci. 1450(1), 147–171 (2019) ArticleCASPubMedPubMed CentralGoogle Scholar
- I. Vályi-Nagy, K.J. Kaffka, J.M. Jákó, É. Gönczöl, G. Domján, Application of near infrared spectroscopy to the determination of haemoglobin. Clin. Chim. Acta 264(1), 117–125 (1997) ArticlePubMedGoogle Scholar
- H. Tian, M. Li, Y. Wang, D. Sheng, J. Liu, L. Zhang, Optical wavelength selection for portable hemoglobin determination by near-infrared spectroscopy method. Infrared Phys. Technol. 86, 98–102 (2017) ArticleCASGoogle Scholar
- Y. Han, J. Chen, T. Pan, G. Liu, Determination of glycated hemoglobin using near-infrared spectroscopy combined with equidistant combination partial least squares. Chem. Intell. Lab. Syst. 145, 84–92 (2015) ArticleCASGoogle Scholar
- M. Paraskevaidi, C.L.M. Morais, D.L.D. Freitas, K.M.G. Lima, D.M.A. Mann, D. Allsop, P.L. Martin-Hirsch, F.L. Martin, Blood-based near-infrared spectroscopy for the rapid low-cost detection of Alzheimer’s disease. Analyst 143, 5959–5964 (2018) ArticleCASPubMedGoogle Scholar
- R.A. Shaw, S. Kotowich, H.H. Mantsch, M. Leroux, Quantitation of protein, creatinine, and urea in urine by near-infrared spectroscopy. Clin. Biochem. 29, 11–19 (1996) ArticleCASPubMedGoogle Scholar
- D.S. Cho, J.T. Olesberg, M.J. Flanigan, M.A. Arnold, On-line near-infrared spectrometer to monitor urea removal in real time during hemodialysis. Appl. Spectrosc. 62(8), 866–872 (2008) ArticleCASPubMedGoogle Scholar
- R. Suzuki, M. Ogawa, K. Seino, M. Nogawa, H. Naito, K. Yamakoshi, S. Tanaka, Reagentless estimation of urea and creatinine concentrations using near-infrared spectroscopy for spot urine test of urea-to-creatinine. Adv. Biomed. Eng. 7, 72–81 (2018) ArticleGoogle Scholar
- F. Zapata, F.E. Ortega-Ojeda, C. García-Ruiz, Revealing the location of semen, vaginal fluid and urine in stained evidence through near infrared chemical imaging. Talanta 166, 292–299 (2017) ArticleCASPubMedGoogle Scholar
- R. Pandey, N.C. Dingari, N. Spegazzini, R.R. Dasari, G.L. Horowitz, I. Barman, Emerging trends in optical sensing of glycemic markers for diabetes monitoring. Trends. Anal. Chem. 64, 100–108 (2015) ArticleCASGoogle Scholar
- T. Monteyne, R. Coopman, A.S. Kishabongo, J. Himpe, B. Lapauw, S. Shadid, E.H. Van Aken, D. Berenson, M.M. Speeckaert, T. De Beer, J.R. Delanghe, Analysis of protein glycation in human fingernail clippings with near-infrared (NIR) spectroscopy as an alternative technique for the diagnosis of diabetes mellitus. Clin. Chem. Lab. Med. 56(9), 1551–1558 (2018) ArticleCASPubMedGoogle Scholar
- Heise HM, Delbeck S, Küpper L (2018) Recent advances in sensor developments based on silver halide fibers for mid-infrared spectrometric analysis. In: Gupta VP (ed). Molecular and Laser Spectroscopy: Adv. Appl. Elsevier, San Diego, Chapter 3, 39–63 Google Scholar
- H.M. Heise, S. Haiber, M. Licht, D.F. Ihrig, C. Moll, M. Stücker, Recent progress in non-invasive diabetes screening by diffuse reflectance near-infrared skin spectroscopy. Proc. SPIE 6093, 609310 (2006) ArticleCASGoogle Scholar
- S. Delbeck, T. Vahlsing, S. Leonhardt, G. Steiner, H.M. Heise, Non-invasive monitoring of blood glucose using optical methods for skin spectroscopy – opportunities and recent advances. Anal. Bioanal. Chem. 411, 63–77 (2019) ArticleCASPubMedGoogle Scholar
- J.T. Olesberg, L. Liu, V. Van Zee, M.A. Arnold, In vivo near-infrared spectroscopy of rat skin tissue with varying blood glucose levels. Anal. Chem. 78, 215–223 (2006) ArticleCASPubMedGoogle Scholar
- Kessoku S, Maruo K, Okawa S, Masamoto K, Yamada Y (2011) Influence of blood glucose level on the scattering coefficient of the skin in near-infrared spectroscopy. Proc. AJTEC2011, paper No AJTEC2011-44471; https://doi.org/10.1115/ajtec2011–44471 Google Scholar
- Heise HM (2000) In vivo Assay of glucose. In: Encyclopedia of Analytical Chemistry: Instrum. Appl. Meyers RA (ed.), Wiley, Chichester Vol. I, 56–83 Google Scholar
- N.V. Alexeeva, M.A. Arnold, Impact of tissue heterogeneity on noninvasive near-infrared glucose measurements in interstitial fluid on rat skin. J. Diabe. Sci. Technol. 4(5), 1041–1054 (2010) ArticleGoogle Scholar
- J. Allen, Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 28, R1–R39 (2007) ArticlePubMedGoogle Scholar
- Y. Yamakoshi, K. Matsumura, T. Yamakoshi, J. Lee, P. Rolfe, Y. Kato et al., Side-scattered finger-photoplethysmography: experimental investigations toward practical noninvasive measurement of blood glucose. J. Biomed. Opt. 22(6), 67001 (2017) ArticlePubMedGoogle Scholar
- W. Nahm, H. Gehring, Non invasive in vivo measurement of blood spectrum by time resolved near-infrared spectroscopy. Sens. Actuators. B. 29, 174–179 (1995) ArticleCASGoogle Scholar
- S.L. Jacques, B.W. Pogue, Tutorial on diffuse light transport. J. Biomed. Opt. 13(4), 041303 (2008) ArticleGoogle Scholar
- T.L. Troy, S.N. Thennadiel, Optical properties of human skin in the near infrared wavelength range of 1000 to 2200 nm. J. Biomed. Opt. 6, 167–176 (2001) ArticleCASPubMedGoogle Scholar
- E. Salomatina, B. Jiang, J. Novak, A.N. Yaroslavsky, Optical properties of normal and cancerous human skin in the visible and near-infrared spectral range. J. Biomed. Opt. 11, 064026 (2006) ArticlePubMedGoogle Scholar
- A. Roggan, J. Beuthan, S. Schründer, G. Müller, Diagnostik und Therapie mit dem Laser. Phys. Blätter. 55, 25–30 (1999) ArticleGoogle Scholar
- J. Qu, B.C. Wilson, Monte Carlo modeling studies of the effect of physiological factors and other analytes on the determination of glucose concentration in vivo by near infrared optical absorption and scattering measurements. J. Biomed. Opt. 2(3), 319–25 (1997) ArticleCASPubMedGoogle Scholar
- K. Maruo, Y. Yamada, Near-infrared noninvasive blood glucose prediction without using multivariate analyses: introduction of imaginary spectra due to scattering change in the skin. J. Biomed. Opt. 20(4), 047003 (2015) ArticlePubMedCASGoogle Scholar
- K.J. Jeon, S.J. Kim, K.K. Park, J.W. Kim, G. Yoon, Noninvasive total hemoglobin measurement. J. Biomed. Opt. 7(1), 45–50 (2002) ArticleCASPubMedGoogle Scholar
- G. Yoon, S.J. Kim, K.J. Jeon, Robust design of finger probe in non-invasive total haemoglobin monitor. Med. Biol. Eng. Compu. 43, 121–125 (2005) ArticleCASGoogle Scholar
- X. Yi, G. Li, L. Lin, Noninvasive hemoglobin measurement using dynamic spectrum. Rev. Sci. Instrum. 88, 083109 (2017) ArticlePubMedCASGoogle Scholar
- T.D. Ridder, B.J. Ver Steeg, B.D. Laaksonen, E.L. Hull, Comparison of spectroscopically measured finger and forearm tissue ethanol concentration to blood and breath ethanol measurements. J. Biomed. Opt. 16(2), 028003 (2011) ArticlePubMedCASGoogle Scholar
- V.R. Kondepati, H.M. Heise, Recent applications of near-infrared spectroscopy in cancer diagnosis and therapy. Anal. Bioanal. Chem. 390, 125–139 (2008) ArticleCASPubMedGoogle Scholar
- L.M. McIntosh, M. Jackson, H.H. Mantsch, R. Mansfield, A.N. Crowson, J.W.P. Toole, Near-infrared spectroscopy for dermatological applications. Vib. Spectrosc. 28, 53–58 (2002) ArticleCASGoogle Scholar
- P. Mehnati, S. Khorram, M.S. Zakerhamidi, F. Fahima, Near-infrared visual differentiation in normal and abnormal breast using hemoglobin concentrations. J. Lasers. Med. Sci. 9(1), 50–57 (2018) ArticlePubMedGoogle Scholar
- A. Bogomolov, U. Zabarylo, D. Kirsano, V. Belikova, V. Ageev, I. Usenov, V. Galyanin, O. Minet, T. Sakharova, G. Danielyan, E. Feliksberger, V. Artyushenko, Development and testing of an LED-based near-infrared sensor for human kidney tumor diagnostics. Sensors 17, 1914 (2017) ArticleCASPubMed CentralGoogle Scholar
- Y. Ozaki, C.W. Huck, M. Ishigaki, D. Ishikawa, A. Ikehata, H. Shinzawa, Near-infrared spectroscopy in biological molecules and tissues, in Encyclopedia of Biophysics, ed. by G.C.K. Roberts, A. Watts (Springer, Berlin, 2018) Google Scholar
- A. Spreinat, G. Selvaggio, L. Erpenbeck, S. Kruss, Multispectral near infrared absorption imaging for histology of skin cancer. J. Biophotonics. 13, e201960080 (2020) ArticleCASPubMedGoogle Scholar
- M.G. Sowa, L. Leonardi, J.R. Payette, Karen M. Cross, K.M. Gomez, J.S. Fish, Classification of burn injuries using near-infrared spectroscopy. J. Biomed. Opt. 11(5), 054002 (2006) ArticlePubMedGoogle Scholar
- M.G. Sowa, W. Chuan Kuo, A.C.T. Ko, D.G. Armstrong, Review of near-infrared methods for wound assessment. J. Biomed. Opt. 21(9), 091304 (2016) ArticlePubMedGoogle Scholar
- J. Hoffmann, D.W. Lübbers, H.M. Heise, Applicability of the Kubelka-Munk theory for the evaluation of reflectance spectra demonstrated for haemoglobin-free perfused heart tissue. Phys. Med. Biol. 43, 3571–3587 (1998) ArticleCASPubMedGoogle Scholar
- A. Zybin, V. Liger, R. Souchon, H.M. Heise, K. Niemax, Examination of the oxygenation state of hemoglobin in a phantom and in-vivo tissue applying absorption balancing with two and three laser wavelengths. Appl. Phys. B. 83, 141–148 (2006) ArticleCASGoogle Scholar
- J. Zalev, L.M. Richards, B.A. Clingman, J. Harris, E. Cantu, G.L.G. Menezes, C. Avila, A. Bertrand, X. Saenz, S. Miller, A.A. Oraevsky, M.C. Kolios, Opto-acoustic imaging of relative blood oxygen saturation and total hemoglobin for breast cancer diagnosis. J. Biomed. Opt. 24(12), 121915 (2019) ArticlePubMed CentralGoogle Scholar
- L.Y. Chen, M.C. Pan, C.C. Yan, M.C. Pan, Wavelength optimization using available laser diodes in spectral near-infrared optical tomography. Appl. Opt. 55(21), 5729–5737 (2016) ArticleCASPubMedGoogle Scholar
- D.T. Delpy, M. Cope, Quantification in tissue near-infrared spectroscopy. Phil. Trans. R. Soc. Lond. B. 352, 649–659 (1997) ArticleCASGoogle Scholar
- A. Curra, R. Gasbarrone, A. Cardillo, C. Trompetto, F. Fattapposta, F. Pierelli, P. Missori, G. Bonifazi, S. Serrant, Near-infrared spectroscopy as a tool for in vivo analysis of human muscles. Sci. Rep. 9, 8623 (2019) ArticlePubMedPubMed CentralCASGoogle Scholar
- G. Malakasioti, S.D. Marks, T. Watson, F. Williams, M. Taylor-Allkins, N. Mamode, J. Morgan, W.N. Hayes, Continuous montoring of kidney transplant perfusion with near-infrared spectroscopy. Nephrol. Dial. Transplant. 33, 1863–1869 (2018) ArticleCASPubMedGoogle Scholar
- P. Karthikeyan, S. Moradi, H. Ferdinando, Z. Zhao, T. Myllylä, Optics based label-free techniques and applications in brain monitoring. Appl. Sci. 10(6), 2196 (2020) ArticleCASGoogle Scholar
- P. Korček, G. Straňák, J. Širc, G. Naulaers, The role of near-infrared spectroscopy monitoring in preterm infants. J. Perinato. 37, 1070–1077 (2017) ArticleGoogle Scholar
- M.A. Yücel, J.J. Selb, T.J. Huppert, M.A. Franceschini, D.A. Boas, Functional near infrared spectroscopy: enabling routine functional brain imaging. Curr. Opin. Biomed. Eng. 4, 78–86 (2017) ArticlePubMedPubMed CentralGoogle Scholar
- F. Scholkmann, S. Kleiser, A.J. Metz, R. Zimmermann, J. Mata Pavia, U. Wolf, M. Wolf, An review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage 85, 6–27 (2014) ArticlePubMedGoogle Scholar
- A. Sudakou, S. Wojtkiewicz, F. Lange, A. Gerega, P. Sawosz, I. Tachtsidis, A. Liebert, Depth-resolved assessment of changes in concentration of chromophores using time-resolved near-infrared spectroscopy: estimation of cytochrome-c-oxidase uncertainty by Monte Carlo simulations. Biomed. Opt. Express. 10, 4621–4634 (2019) ArticleCASPubMedPubMed CentralGoogle Scholar
- Y. Mendelson, Pulse Oximetry: Theory and Applications for Noninvasive Monitoring. Clin. Chem. 38, 1601–1607 (1992) ArticleCASPubMedGoogle Scholar
- M. Mehrabi, S. Setayeshi, S.H. Ardehali, H. Arabalibeik, Modeling of diffuse reflectance of light in heterogeneous biological tissue to analysis of the effects of multiple scattering on reflectance pulse oximetry. J. Biomed. Opt. 22(1), 015004 (2017) ArticleGoogle Scholar
- G.S. Hong, A.L. Antaris, H. Dai, Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 1, 0010 (2017) ArticleCASGoogle Scholar
- S. Luo, E. Zhang, Y. Su, T. Cheng, C. Shi, A review of NIR dyes in cancer targeting and imaging. Biomaterials 32, 7127–7138 (2011) ArticleCASPubMedGoogle Scholar
- X.H. Chang, J. Zhang, L.H. Wu, Y.K. Peng, X.Y. Yang, X.L. Li, A.J. Ma, J.C. Ma, G.Q. Chen, Research progress of Near-Infrared Fluorescence Immunoassay. Micromachines 10, 422 (2019) ArticlePubMed CentralGoogle Scholar
- X. Meng, J. Zhang, Z. Sun, L. Zhou, G. Deng, S. Li, W. Li, P. Gong, L. Cai, Hypoxia-triggered single molecule probe for high-contrast NIR II/PA tumor imaging and robust photothermal therapy. Theranostics 8(21), 6025–6034 (2018) ArticleCASPubMedPubMed CentralGoogle Scholar
Acknowledgements
I am tremendously grateful for the collaboration and enormous support from my collaborator Sven Delbeck over many years at the Interdisciplinary Center for Life Sciences in Iserlohn during research and for teaching. Some earlier work was done at the Leibniz-Institute for Analytical Sciences-ISAS, my former research institute in Dortmund. I am grateful for the productive working atmosphere, which had been very much appreciated.
Author information
Authors and Affiliations
- Interdisciplinary Center for Life Sciences, South-Westphalia University of Applied Sciences, Frauenstuhlweg 31, 58644, Iserlohn, Germany Herbert Michael Heise
- Herbert Michael Heise



